Hello everyone,
I hope you are all well, and that you will continue to be well for decades to come. In today’s letter, I will be telling you all about the quest to ensure this happens, that is; the science behind what we have done to address the problem aging and the philosophy as to why this is worthwhile in the first place.
Before diving in, there is something I would like to share with you.
On the website, the number one suggestion I have received was to make these very letters more prominent on the homepage. This would make it easier to access and share them as well as make it clear that the site is frequently updating.
So I redesigned the home page of the site: Please have a look
There will be changes on phones and tablets as well, but for the most significant ones have a look on your desktop (or laptop).
I’m quite pleased with how it turned out.
Lifespan ≠ Healthspan
In the last three hundred years, the average life expectancy across the world has risen from about 30 to 71. In the developed world, this number is 81.
These are conservative numbers. That 71 includes people from the poorest part of the world; it also contains infants that die in childbirth. By virtue of being alive, it is not unreasonable for you to expect to live longer than either of these figures. These are only averages; you could be more unfortunate — or much more fortunate — than this.
But the biggest blind spot of the numbers is that they are based on the world as it is today — it ignores any advancements we make in the field of human health and aging. The truth is it is reasonable to expect to make many breakthroughs here within our lifetimes and that we could all be living a lot longer than this.
A reasonable critique is this; why have the concern over the lifespan in the first place given that longer lives also means more time spent in older age suffering the vicissitudes of illness and physical pain?
Hence, anti-aging researchers have coined the concept of a healthspan as opposed to a mere lifespan.
A healthspan is how many years of life we experience as young, healthy individuals.
With a focus on healthspan, increases in our longevity will mean more years living as we please and doing as we like — rather than merely extending the duration of our time in a nursing home, hospice or medical care.
Not only must we consume the elixir of immortality, but we must also drink from the fountain of eternal youth.
Why we grow old: The causes of aging
I am sure you have heard the traditional explanation of this. In every one of our cells is the chemical code for our entire genetic make-up; our DNA, that is; Deoxyribonucleic Acid. Every time a cell replicates, it was believed that this DNA was often copied imperfectly. Much like when you photocopy an image over and over, the quality of the replication dulled over time. Over time these mistakes accumulate, the errors in the copying add up, and our cells are increasingly damaged. This is what we believed was the reason behind our aging.
But it is not quite right.
The conventional theory — that aging is damage accumulated in cells due to errors in replicating the DNA — we now know to be false.
When we take an adult cell, which has already replicated many times and use it to engineer a clone, they have the same lifespan as a natural organism. This would suggest that the quality of information in their DNA is just as high. There has not been a build-up of errors in the genome as we would expect if the theory were true.
Cloning, by the way, is a technology that has been around for some time. The first clone we engineered was Dolly the Sheep which we created in 1996. Today the singer Barbara Streisand has clones of her deceased pet dog.
If these clones can live just as long as their natural counterparts, then the quality of the information in their genes has not been compromised — it means there must be another explanation for why we age over time.
The damage that we suffer that causes us to age is not in the genome. David Sinclair, an Australian Biologist, professor of genetics and a leading researcher into the problem of aging believes that it is in our epigenome.

Remember how I said that a complete copy of our genetic instructions was in each and every cell in the body?
But not every cell needs all of this information.
The liver cell does not need to know how to deal with neurotransmitters, and white blood cells do not need to know how to make bone marrow.
A complete copy of the genome is in each and every cell in your body — yet, no cell needs all of the instructions within it.
The epigenome is responsible for determining which parts of the genome are used in any given cell. That is; which sections of the DNA sequence are active and inactive.
It does this using proteins called histones which the DNA strands are wrapped on, through a process called methylation. How it deactivates genes includes coiling up unneeded parts of the DNA sequence around the histones and through methylation, by attaching chemical markers which turns off specific parts of the sequence.
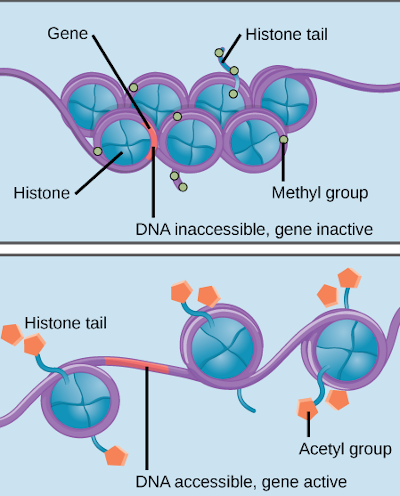
It is because of the epigenome that a skin cell “knows” it is a skin cell, an eye cell “knows” it is an eye cell, and so on so forth. If the epigenome is damaged, a cell will not know what it is supposed to do and fail to function.
“Weird stuff happens when you get older, you start getting hair growing on your ears, nose and back. That’s cells losing their identity.”
David Sinclair, on Veritasium
According to David, over time, it is our epigenome that decays and our cells “forget” their knowledge of what type of cell they are. It is this loss of their original functionality that is responsible for what we call aging.
When DNA gets damaged, the rest of the cell steps into gear to rebuild it. Most of the time, this works out fine. But about 1% of the time this happens, the parts of the epigenome do not return to the right place. The histones are no longer in the right place, and the methylation which blocked parts of the cell from working are no longer in the correct sequence. These are the errors that accumulate over time that we call aging.
These irregularities in the methylation accumulate on the DNA strand, “a bit like plaque on the teeth”. Over time, as the errors build-up, more and more of this happens. We can measure the bits of epigenetic plaque on the gene to calculate a person’s biological age.
“We call it the Horvath clock after my good friend Steve Horvath.”
David Sinclair, the same video as above
Putting the epigenetic theory of aging to the test, David and his colleagues ran experiments on lab mice and were able to make them age faster. The epigenome steps in to repair the DNA every time it gets damaged, so what they did was intentionally damage the genome of these mice over and over throughout their lives. This was done carefully; the DNA was not damaged beyond repair — to the point of mutation — so all of the genetic information was preserved (the cells restored the DNA perfectly). The epigenome, however, was put under a lot of pressure and withered over time.
In the end, the mice did age as expected. Once again:
- There was no genetic damage
- There was damage to the epigenome
They grew grey hairs, became hunched, moved more slowly and the DNA clock showed that they aged fifty per cent faster.
SENS and Gandalf Aubrey de Grey

The medical term for aging is senescence. The term negligible senescence was first used to describe creatures such as lobsters that seem to show no signs of aging. Strategies for Engineered Negligible Senescence is a term coined by the Biomedical Gerontologist[1] Aubrey de Grey. If lobsters and certain types of jellyfish have natural negligible senescence — that is; negligible aging — we can have engineered negligible senescence. We can look into the causes of damage to our physical make-up at the cellular level and address each of these concerns.
“The aging of a human being, or any living organism, is actually really similar, really very similar, to the aging of an inanimate object like a car or an airplane. It’s simply the accumulation of damage as a side effect of the machine’s normal operation.”
Aubrey de Grey
Aubrey’s view on aging is not synonymous to that of David’s. According to him, through the normal operation of our bodies, we accumulate cellular damage through our lives — much in the same way that a knife dulls over time, or a car breaks down. Addressing the problem of aging is synonymous with identifying and fixing the types of cellular damage before it builds up.
Aubrey identifies the full list of damage as follows:
- Cell loss
- Cancerous cells
- Mitochondrial mutations
- Death-resistant cells
- Extracellular matrix stiffening
- Extracellular aggregates
- Intracellular aggregates
I will not try to explain any of these here - for I do not even know what some of them mean - but the point here is that there are a finite number of causes to the range of phenomena we call aging.
Exilirs of Immortality: Solutions to the problem
We tend to view life as though it followed a natural arc. Birth, childhood, adolescence, adulthood, old age, sickness and death. As if the natural course of events was an inevitable one, a rite of passage, a journey we all had to pass through.
But this is simply not the case. As David Deutsch has said; all problems are solvable.
In fact, we have already altered the natural course. Only a few centuries ago, the average lifespan was about thirty years. The natural course of life should probably entail being mauled by a bear, dying of the common cold, an infection from an ordinary wound or in childbirth. That is; if we judge what is natural by the vast majority of human history.
Just as thirty was an arbitrary number, eighty is also. There’s no reason why we can’t modify the so-called natural course of events to the benefit of us all.
To give you an idea of how tractable these problems are; lobsters and certain types of jellyfish already do not age.
Whatever the solution is, something happens to our bodies that doesn’t need to happen. We simply need to figure it out.
There is too much research in anti-aging to summarise in this letter, so I will briefly cover three breakthroughs.
Tapping into our Anti-Aging genes
According to David Sinclair, there are sets of genes we already have known as the hormetic response genes which fight against the processes that are responsible for aging. They do this in several ways, but the most important to David, is that they protect and maintain the epigenome.
These are ancient genes that have existed since we were single-celled organisms. In ideal environments, the normal genes for growth and reproduction were activated, but in harsher environments where this was not possible genes for protecting and maintaining the cell were enabled instead. These are what we consider the anti-aging genes.
Basically, the idea is by exposing our body to levels of stress we can activate these genes, which make our epigenome last longer and therefore slow the aging process.
One of the most reliable ways to make lab mice live longer is to feed them less. When they are receiving less food, the body believes it is in a condition of scarcity. Rather than organising its systems for growth and development, it will enter the “protect and maintain” mode and release the longevity genes.
Caloric restriction as a way of increasing lifespan has been tried and tested in several animal trials, including on monkeys which are much more similar to us than mice.
“They didn’t just age slower, they didn’t get as much diabetes and heart disease.”
David Sinclair on the trials on caloric restrictions on Monkeys, here again
A few weeks ago, I attended an online Think Inc event where David presented his case for being able to address the problem of aging. David himself endorses caloric restriction as the best thing a person can do apart from obvious big five; don’t smoke, don’t drink much, eat healthy, exercise regularly and maintain a healthy body weight. David himself lives on one meal a day, only drinking coffee in the morning and just having dinner.
Five recommendations he gives are:
- Eating less
- Avoiding DNA damage (apply sunscreen, only take x-rays if there is good reason)
- Eating less protein (makes the body feel like it is full, and deactivates longevity genes)
- Intense but brief physical exercise (trick your body into thinking it is running from a bear)
- Allowing yourself to feel uncomfortably hot/cold (stress on the cells activates the genes)
He was also quite enthusiastic about suggesting exercise while hungry. He says that when you get used to these behaviours, they are not nearly as bad as they sound.
Happily, for those of you like myself — to whom these steps seem more than a little unpleasant — there is another way to activate these anti-aging genes.
There is a chemical that exists naturally in our cells called NAD+ that activates our longevity genes. It is not possible to introduce NAD+ into our cells as it would change its structure as it passes through the cell wall, but it is possible to add a chemical called NMN which raises the levels of NAD+ inside the cell.
When David and his team injected mice with NMN and tested their fitness, they found they had to reprogram their treadmill machines...
“The program had never seen a mouse that ran more than three kilometres.”
“Three Kilometres?! For a mouse?”
“For an old mouse.”
Frankenstein’s pet mouse
In 2012, the Japanese Scientist Shinya Yamanaka discovered a way of resetting an adult cell’s epigenome back to its state as an embryo. The four factors that allow us to do this are now called the Yamanaka factors.
Think about this for a moment.
- According to David Sinclair, the cause for aging is damage dealt to the epigenome.
- It is possible to reset the epigenome back to how it was at the beginning
Not only is it possible to slow down aging, we can be more ambitious — we can reverse it.
David and his team were this ambitious. They attempted applying three of these Yamanaka factors on the eyes of older mice who had lost their vision. The reason they chose the eyes, is because when it comes to aging, we consider eye damage the hardest to repair.
“We put the gene therapy in the eyes of old mice, it turned their retinas young again — those mice could see again.”
David Sinclair
Nothing about this therapy is specialised to the eye though, there is no reason this could not be done with every cell and at least in principle reset their ages.
Not only can we slow aging, there may well come a day when we will be able to reverse it.
Resources
There is a lot more to this field I could cover, but there is only so much a letter can say. Here are some more resources into this, including talks by Aubrey de Grey and David Sinclair. The videos by Derek Muller (Veritasium) were especially useful for me here.
-
How to cure aging — during your lifetime by Kurzgesagt – In a Nutshell (~ 7 minutes)
-
Aubrey de Grey’s 2005 TED Talk: A roadmap to ending aging (23 minutes)
-
David Sinclair’s 2013 TEDxSydney Talk: A cure for ageing? (16 minutes)
-
Derek Muller interviewing David Sinclair for Veritasium (21 minutes)
-
The SENS Research Foundation: Aubrey de Grey is the Chief Scientific Officer here, SENS is dedicated to addressing the problem of ageing rather than its symptoms such as heart disease, Alzheimer’s and diabetes
-
Lifespan.io: For all the latest news about developments in aging resource, the most comprehensive place I know of for this. Aubrey de Grey himself endorses it.
So we’ve come to the end of this letter, this one has turned out to be over 2700 words. I wonder what the secret to its longevity is. I want to close by briefly mentioning the philosophical implications behind all this. It should be clear that we will one day be in the position to address the deeper causes of what we call aging and radically improve our healthspan.
But should we?
In my view, the answer is: Yes, obviously.
But I feel I have run out of words, so the philosophy behind this will have to wait until a future letter.
In the meantime, I heartily encourage all of you to read this epic story by the super-intelligent Nick Bostrom.
The Fable of the Dragon-Tyrant
It is honestly amazing, and I hope you gain as much value out of it as I have.
And with that, I will bid you adieu — if you have read this far, I am more grateful to you than you realise.
Live long and prosper,
Sashin
Gerontology is the study of aging. Not just the biological but the social, cultural, cognitive and psychological aspects of it too. ↩︎
Discussion